ISHIGAKI Yusuke
Associate Professor
Creating Fundamental Chemistry Based on Our Original Molecular Design
Department of Chemistry, Organic and Biological Chemistry

| Theme | Preparation of Redox-active Highly Strained Molecules with a Novel Functionality |
| Field | Structural Organic Chemistry, Organic Chemistry, Physical Organic Chemistry |
| Keyword | Highly strained compounds, Redox systems, Electrochromism, Mechanochromism, Long bond, Hyper covalent bond |
Introduction of Research
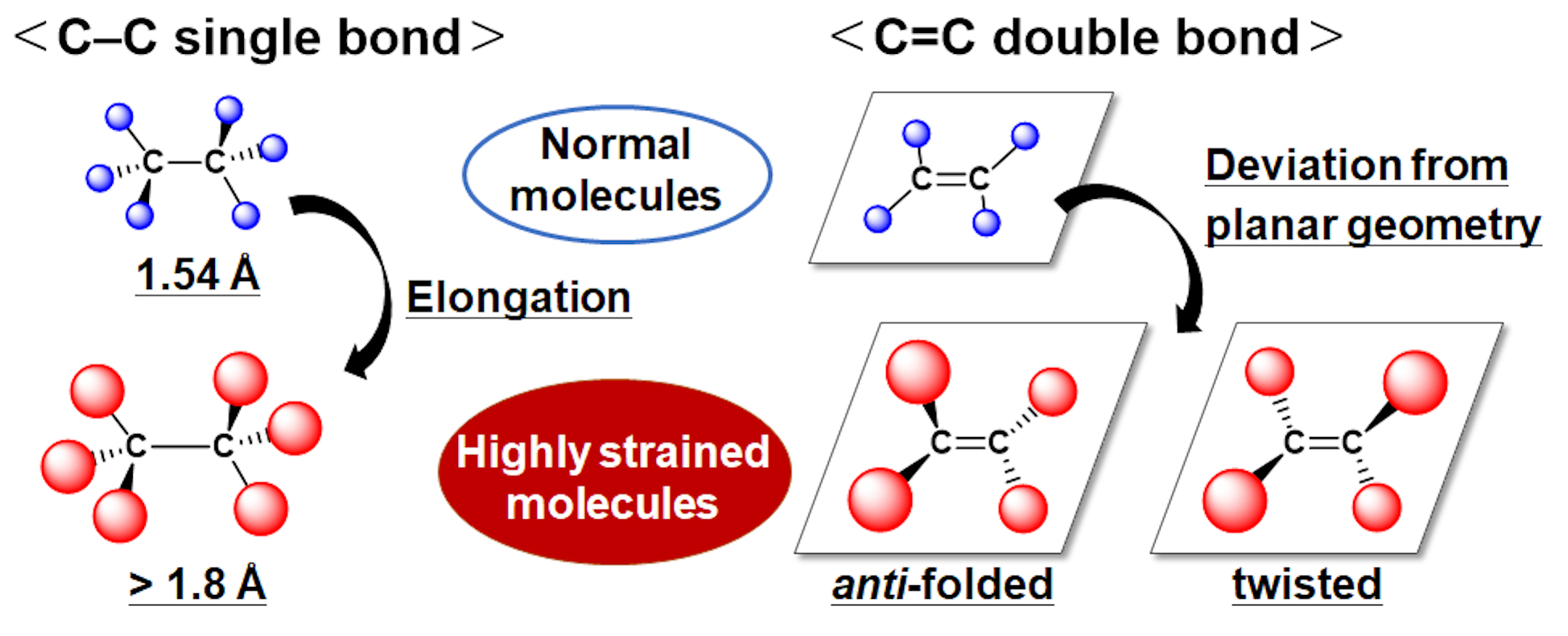
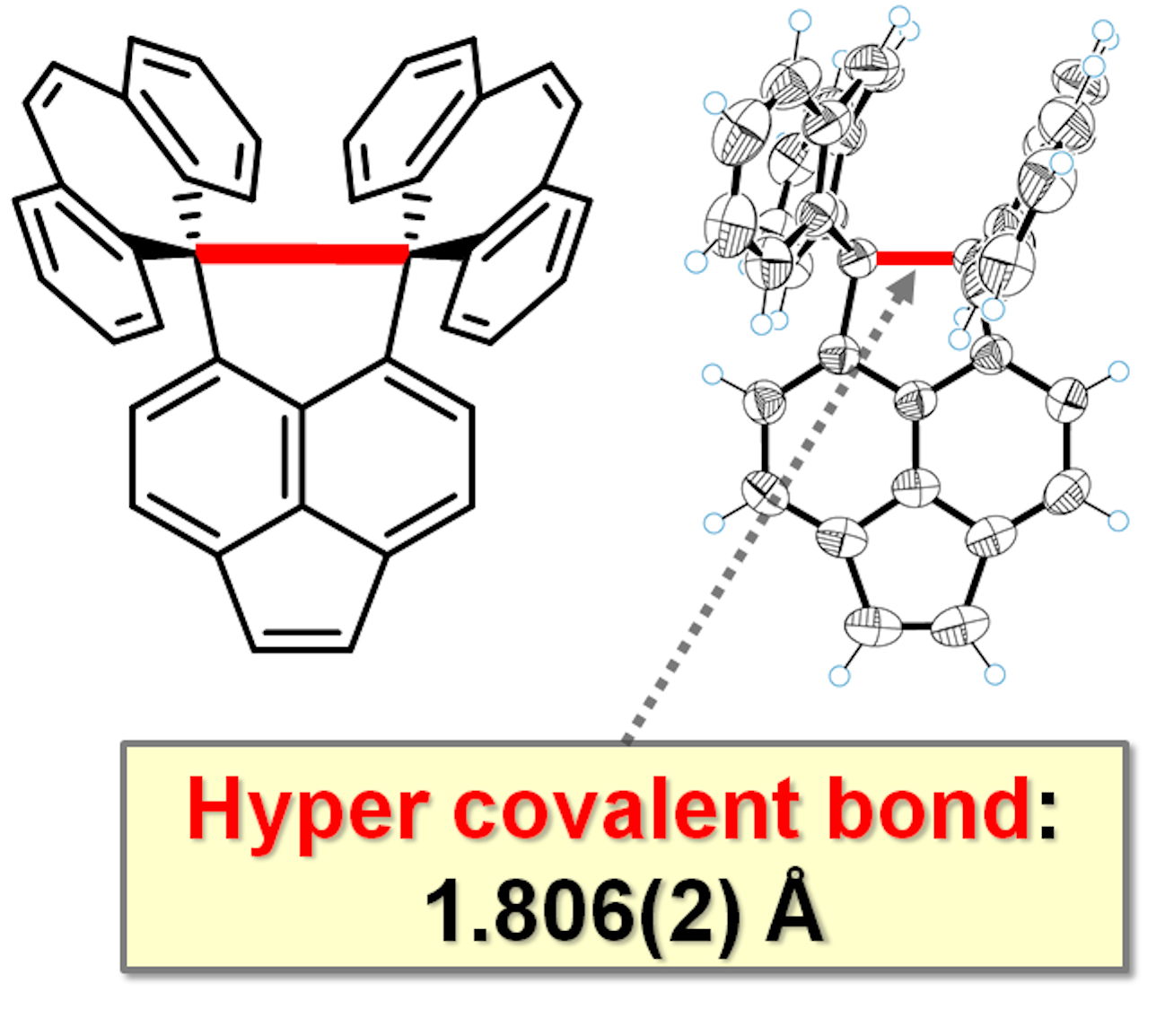
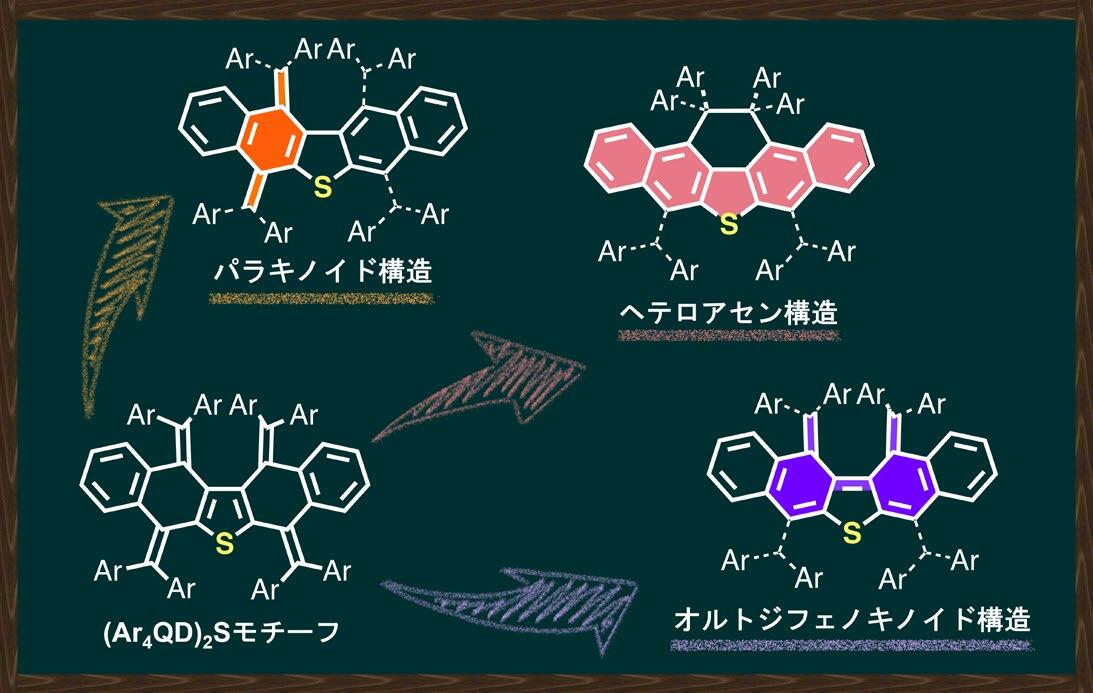
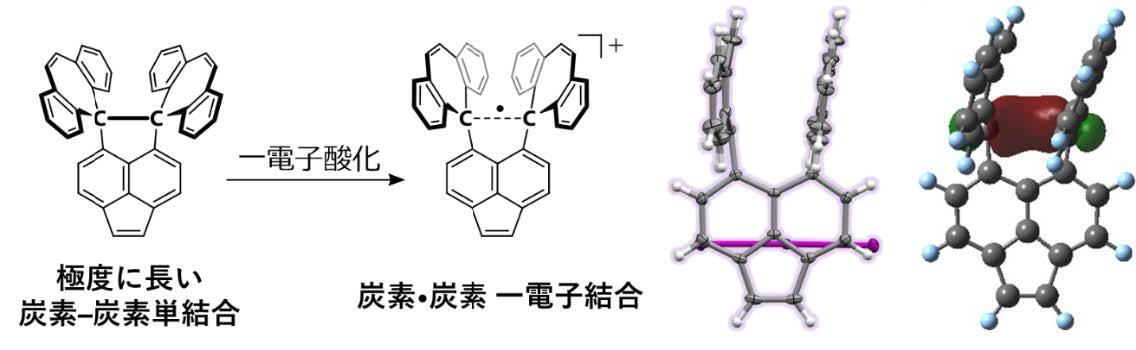
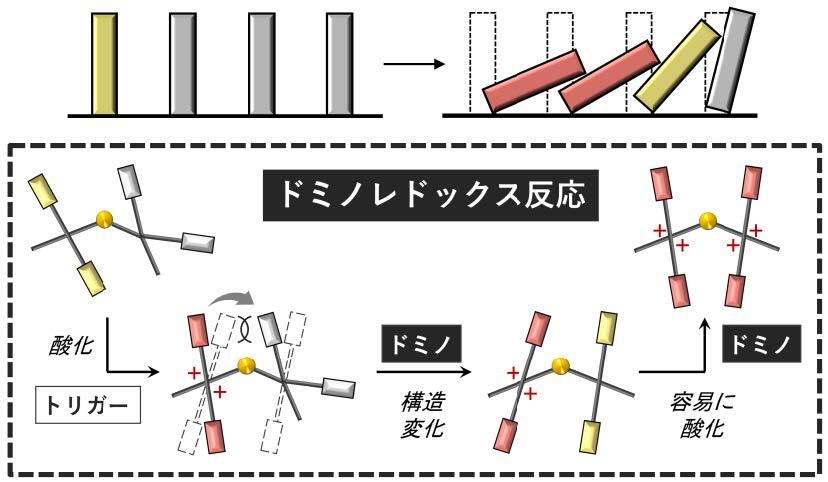
The carbon–carbon covalent bond is one of the most basic concept in organic chemistry. Bond length, bond angle, and torsion angle among carbon atoms are nearly constant on the basis of their hybrid orbitals. On the other hand, highly strained hydrocarbons such as sterically-congested and/or curved polycyclic aromatic hydrocarbons as well as cyclic π-conjugated molecules have attracted much attention with regard to their characteristic features. We focused on two types of redox-active molecules with a extremely elongated C-C single bond (Hyper covalent bond with a bond length beyond 1.8 Å) or a strained double bond, for which unique electrochromic behavior was observed upon oxidation to give deeply colored cationic species.
Representative Achievements
T. Shimajiri, S. Kawaguchi, T. Suzuki, Y. Ishigaki
Nature 2024, 634, 347-351. (Chem-Station Spotlight Research No. 627)
Y. Hayashi, S. Suzuki, T. Suzuki, Y. Ishigaki
J. Am. Chem. Soc. 2023, 145, 2596-2608. (Chem-Station Spotlight Research No. 501)
Y. Ishigaki, T. Harimoto, K. Sugawara, T. Suzuki
J. Am. Chem. Soc. 2021, 143, 3306-3311. (Chem-Station Spotlight Research No. 308)

Related industries
| Academic degree | Ph.D. |
| Academic background | 2008 B.S. Department of Chemistry, Faculty of Science, Hokkaido University 2009 M.S. Department of Chemistry, Graduate School of Science, Hokkaido University 2012 Ph.D. Department of Chemistry, Graduate School of Science, Hokkaido University 2012-2013 JSPS Research Fellow (PD), Ulm University, Germany 2013-2015 Nippon Steel & Sumikin Chemical Co., Ltd. 2016- Assistant Professor, Department of Chemistry, Faculty of Science, Hokkaido University 2021年- Associate Professor, Department of Chemistry, Faculty of Science, Hokkaido University |
| Affiliated academic society | The Chemical Society of Japan, The Society of Physical Organic Chemistry, Japan, The Society of Synthetic Organic Chemistry, Japan, The Japanese Photochemistry Association |
| Room address | Science Building 6 #6-509 |