A redox reaction triggered by hydrostatic pressure in dicationic cyclophanes
Joint press release (in Japanese) by Hokkaido University and Kyushu University
Abstract
Various reactions and systems that respond to hydrostatic pressure, i.e., one type of mechanical isotropic stimulus, have been developed over the past decades. Here, we show that a one-electron (1e) reduction of dicationic cyclophane can be realised by applying hydrostatic pressure in a water-containing solvent. The large negative value of the volume change 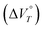 observed for this reduction, which is key to inducing the reduction reaction, is due to the desolvation of the H2O molecules and the change in the proximity between the cyclophane π units accompanied by a decrease in electrostatic repulsion. In fact, related monocations did not undergo a 1e reduction under hydrostatic pressure, even in water-containing solvents, indicating that the reduction behaviour is enabled by the cyclophane structure. Furthermore, in the case of weakly polar anions such as BF4− and PF6−, ….
observed for this reduction, which is key to inducing the reduction reaction, is due to the desolvation of the H2O molecules and the change in the proximity between the cyclophane π units accompanied by a decrease in electrostatic repulsion. In fact, related monocations did not undergo a 1e reduction under hydrostatic pressure, even in water-containing solvents, indicating that the reduction behaviour is enabled by the cyclophane structure. Furthermore, in the case of weakly polar anions such as BF4− and PF6−, ….
Read the original article on Materials Chemistry Frontiers
Article inforamation
Moto Kikuchi, Tomoya Kuwabara, Gaku Fukuhara, Takanori Suzuki and Yusuke Ishigaki, A redox reaction triggered by hydrostatic pressure in dicationic cyclophanes, Materials Chemistry Frontiers, 2025
DOI:10.1039/D5QM00426H