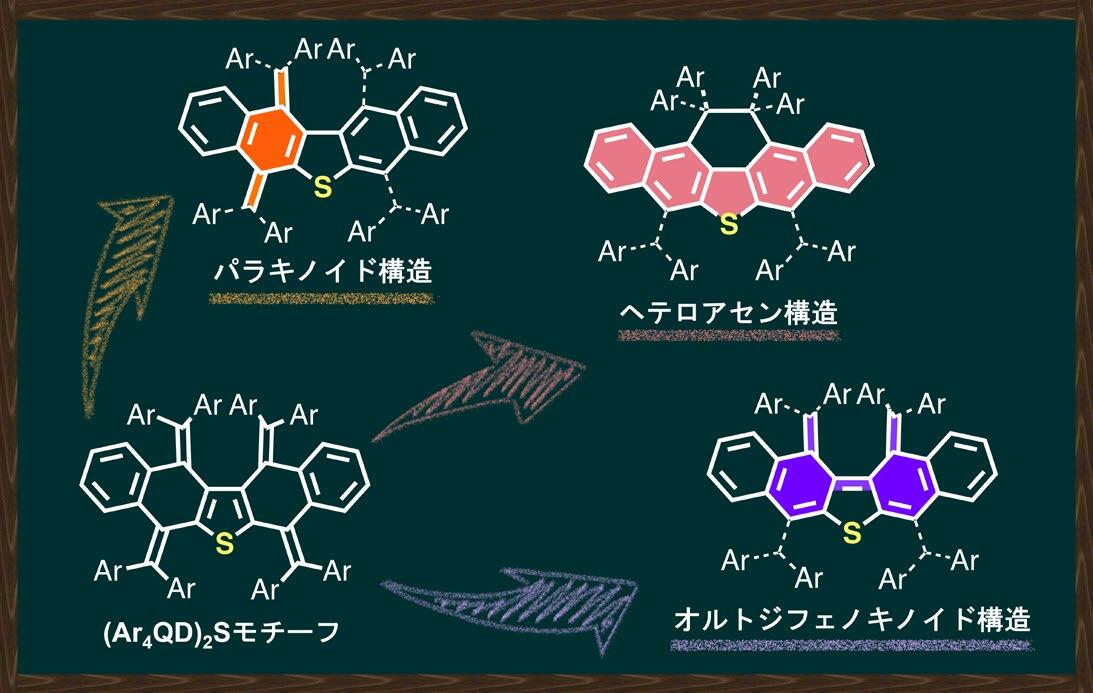
Diverse redox-mediated transformations to realize the para-quinoid, σ-bond, and ortho-diphenoquinoid forms
Abstract
π-Electron systems with multiple redox-active units have attracted attention in various fields due to their potential applications. However, the design strategy remains elusive to selectively synthesize the diverse molecular structures of redox-convertible species. In this study, covalently linked quinodimethane derivatives with a sulfur bridge [(Ar4QD)2S] were designed as redox-active motifs that can be converted into three different geometries via redox reaction. Here we show that the favored geometry of the corresponding redox states of (Ar4QD)2S can be precisely controlled by adjusting the steric bulk of the substituents on the aryl group to change the proximity of the quinodimethane units. Notably, this redox-mediated strategy also leads to ……
Read the original article on Nature Communications
Article inforamation
Harimoto, T., Kikuchi, M., Suzuki, T. et al. Diverse redox-mediated transformations to realize the para-quinoid, σ-bond, and ortho-diphenoquinoid forms. Nat Commun 16, 4088 (2025).
DOI: 10.1038/s41467-025-59317-w