Switching of Redox Properties Triggered by Thermal Equilibrium between Closed shell Folded and Open shell Twisted Species
Abstract
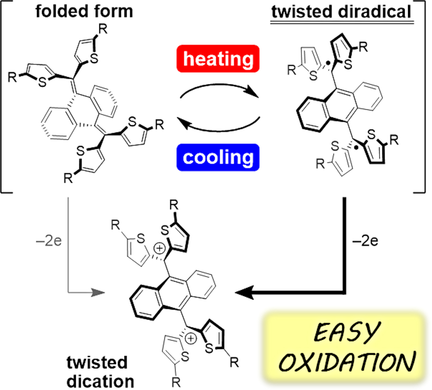
Thermally switchable redox properties have been reported to be due to a change in the spin state of newly designed overcrowded ethylenes, which can adopt closed‐shell folded and open‐shell twisted forms. In this study, tetrathienylanthraquinodimethane derivatives were designed to be in thermal equilibrium between a more stable folded form and less stable but more donating twisted diradical in solution, so…..
Read more on Angewandte Chemie
Article Information:
Switching of Redox Properties Triggered by Thermal Equilibrium between Closed shell Folded and Open shell Twisted Species
Y. Ishigaki, T. Hashimoto, K. Sugawara, S. Suzuki, T. Suzuki
Angew. Chem. Int. Ed., in press.
DOI: 10.1002/anie.201916089