Deubiquitinating enzymes UBP12 and UBP13 stabilize the brassinosteroid receptor BRI1
Abstract
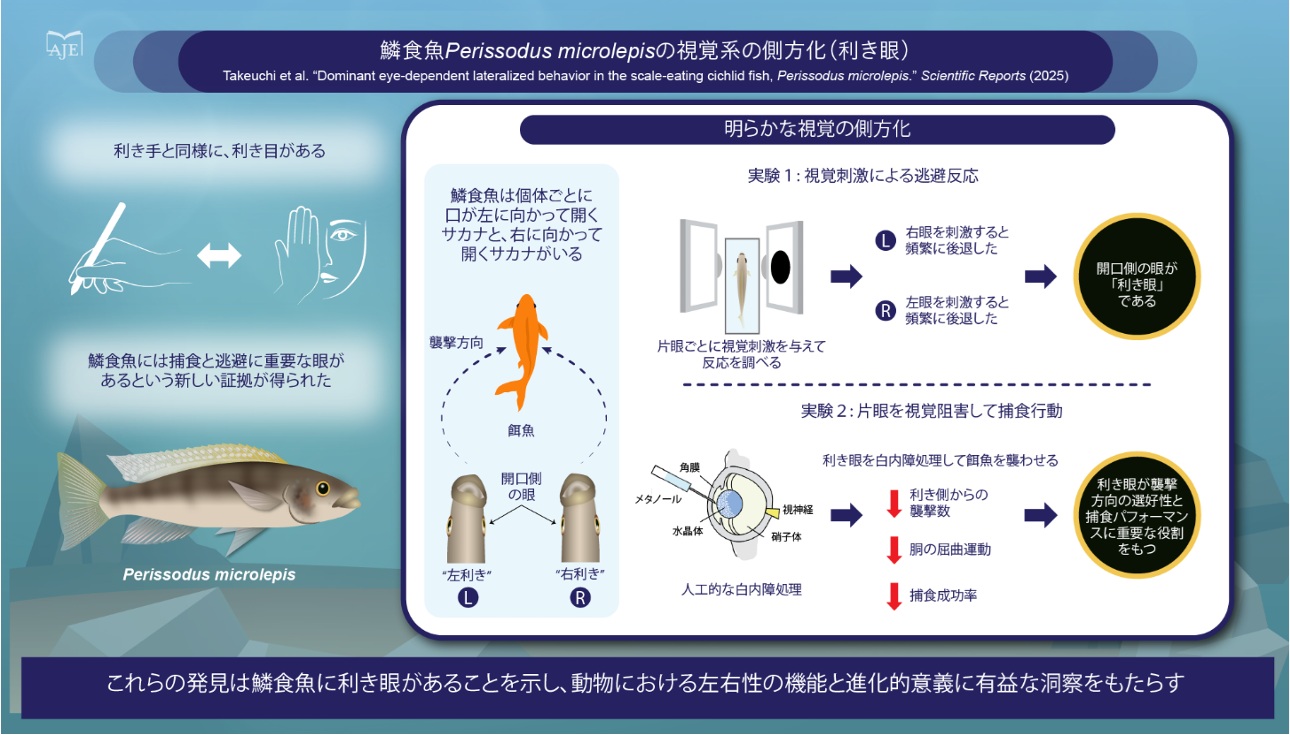
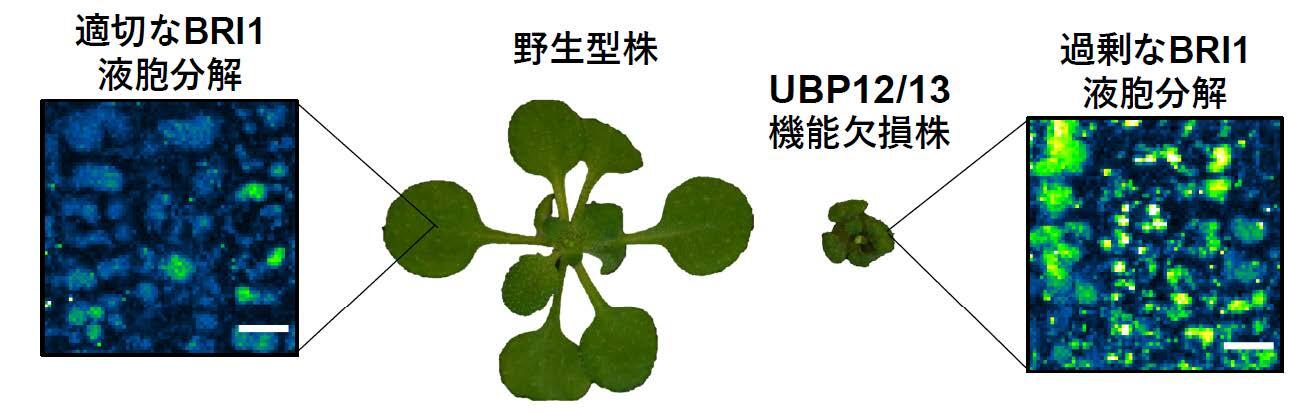
Protein ubiquitination is a dynamic and reversible post-translational modification that controls diverse cellular processes in eukaryotes. Ubiquitin-dependent internalization, recycling, and degradation are important mechanisms that regulate the activity and the abundance of plasma membrane (PM)-localized proteins. In plants, although several ubiquitin ligases are implicated in these processes, no deubiquitinating enzymes (DUBs), have been identified that directly remove ubiquitin from membrane proteins and limit their vacuolar degradation. Here, we discover……
Read the original article on EMBO reports
Article information:
Yongming Luo, Junpei Takagi, Lucas Alves Neubus Claus, Chao Zhang, Shigetaka Yasuda, Yoko Hasegawa, Junji Yamaguchi, Libo Shan, Eugenia Russinova, Takeo Sato,
Deubiquitinating enzymes UBP12 and UBP13 stabilize the brassinosteroid receptor BRI1
EMBO Reports (2022)e53354
DOI: 10.15252/embr.202153354