SAKAGUCHI Kazuyasu
Specially Appointed Professor
Understand fundamental principle and interpretation of life phenomena in the chemical points of view
Department of Chemistry, Organic and Biological Chemistry

| Theme | "Function, structure, and regulation of tumor suppressor protein p53 and related factors" |
| Field | Biological Chemistry, Functional Biochemistry, Structural Biochemistry, Molecular Biology, Cellular Biology, Protein Science, Peptide Science |
| Keyword | Tumor Supperessor Protein, Cell Cycle, Differentiation, Canser, Evolution, Oligomerization, Phosphatase, Phosphorylation/Dephospharylation, Anticancer drug, Bionanomaterials |
Introduction of Research
Function, oligomerization, regulation and evolution of the tumor suppressor protein p53
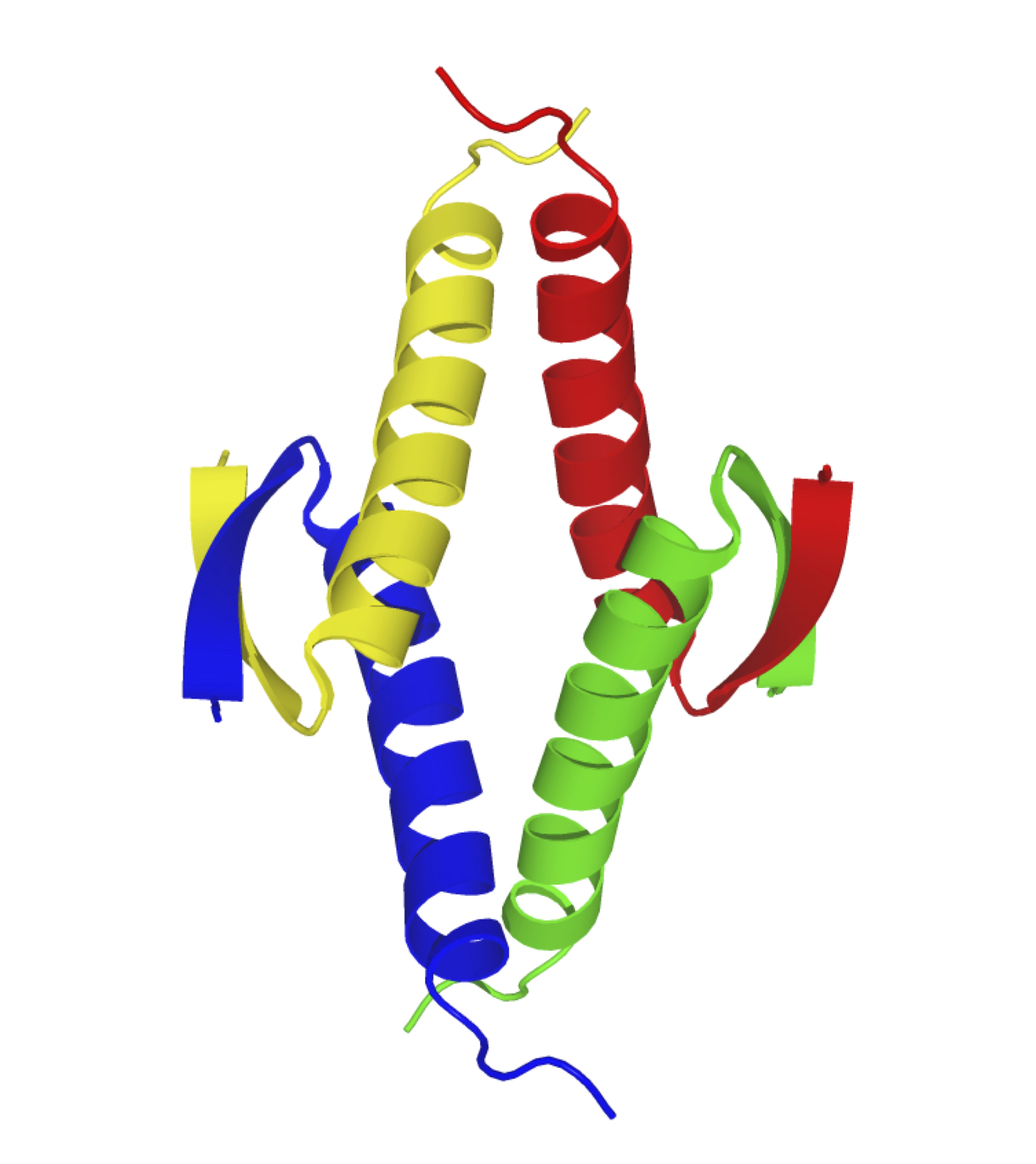
The tumor suppressor protein p53 is activated in response to a wide variety of cellular stresses including DNA damages and starvation in order to maintain “genomic integrity” through induction of cell cycle arrest and apoptosis. The tetramer formation of p53 is essential for its functions. The TP53 gene, which encodes p53, is the most frequently mutated gene in human malignant tumors. In our laboratory, we are pursuing detailed research on the regulatory mechanisms of the function and tetramerization for p53 by post-translational modification and interaction with other factors. We are also interested in evolution of the p53/p63/p73 family.
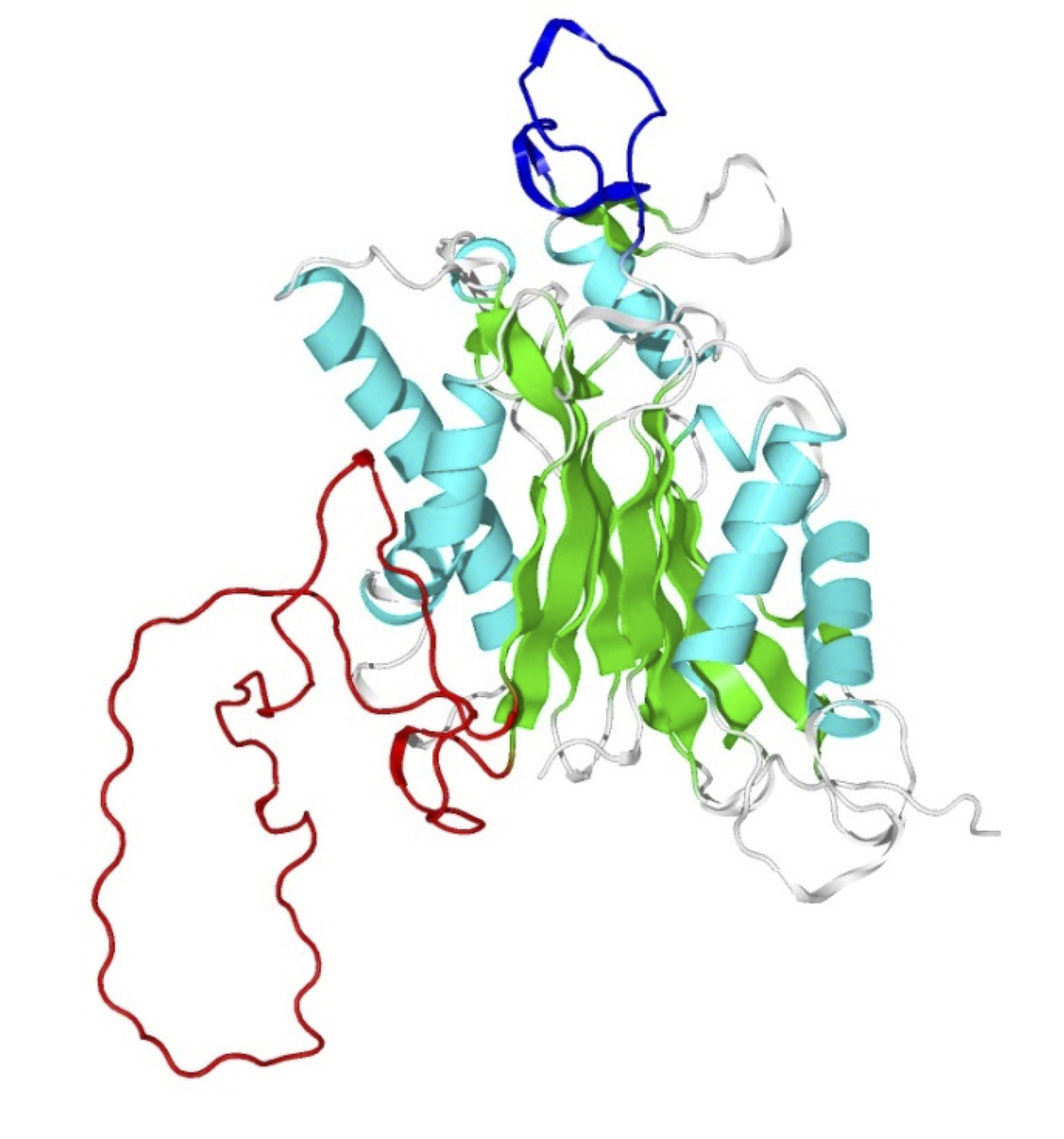
Function and substrate recognition by phosphatase PPM family phosphatases
Phosphorylation by kinases and dephosphorylation by phosphatases is the most important post-translational modification of proteins in the regulation for cellular responses. PPM family proteins are Mg2+/Mn2+-dependent Ser/Thr phosphatases involved in various cellular responses. Aberrant functions of PPM family cause failures in phosphorylation/dephosphorylation signaling, resulted in various illnesses, including cancer, neurological disorders, and metabolic diseases. Our goal is to understand the functions, regulatory mechanisms, and substrate recognition of PPM phosphatases. We are also developing highly potent and specific PPM1D inhibitors as an anti-tumor drug
Pursuit of the principle of life: Understanding the Central dogma of molecular biology and the interaction between mirror image biomolecules
In cells, genetic information is preserved by DNA replication, DNA information is transcribed into mRNA, and polypeptides (proteins) are biosynthesized by translation based on the information of mRNA. This framework is called the Central Dogma of Molecular Biology and is treated as the basis of the principle of life. Understanding how the first life was formed and evolved is one of the most important goals of life science. In our laboratory, we are conducting research study to elucidate the synthetic pathway and its function of polypeptides in bacteria, which deviates from the principle of central dogma.
Proteins and DNA consist of L-amino acids and D-form deoxyribose, respectively. It is not yet clear why these optical isomers were selected in the origin of life. It is of great interest to clarify the cross-reactivity of cognitive abilities between enantiomer molecules. We are conducting research to understand specific interactions between natural and unnatural enantiomeric forms of biomolecules.
Representative Achievements
Milosevic J, Treis D, Fransson S., Gallo-Oller G., Sveinbjörnsson B., Eissler N., Tanino K., Sakaguchi K., Martinsson T., Wickström M., Kogner P., Johnsen JI., Cancers, 13, 6042 (2021)
Kamada R., Kimura N., Yoshimura F., Tanino K., Sakaguchi K., PLOS ONE, 14, e0212682 (2019)
Sakaguchi T., Janairo, J. I. B., Lussier-Price M. Wada J., Omichinski J.G., Sakaguchi K. Scientific reports 7: 1400 (2017)
Kamada R., Toguchi Y., Nomura T., Imagawa T., Sakaguchi K. Biopolymers, 106, 598 (2016).
Kozakai Y., Kamada R., Furuta J., Kiyota Y., Chuman Y., and Sakaguchi K. Scientific reports 6, 33272 (2016)
Related industries
| Academic degree | Ph.D. |
| Academic background | 1983 B.Sc. Kyushu University, Fukuoka, Japan, 1986 M.Sc. Kyushu University, Fukuoka, Japan, 1989 Ph.D. Kyushu University, Fukuoka, Japan 1989 Visiting Fellow National Cancer Institute, National Institutes of Health, MD, USA, 1992 Visiting Associate National Cancer Institute, National Institutes of Health, MD, USA, 1997 Staff Scientist Appointed National Cancer Institute, National Institutes of Health, MD, USA, 1999 Staff Scientist National Cancer Institute, National Institutes of Health, MD, USA, 1999 Associate Professor Kyushu University, Fukuoka, Japan, 2003 Professor Hokkaido University, Sapporo, Japan, |
| Affiliated academic society | The Japanese Biochemical Society, The Chemical Society of Japan, The Japanese Peptde Society, Japanese Association For Protein Phosphatase Research, Japanese Society for Chemical Biology, American Chemical Society |
| Room address | Science Building 6 Room 6-5-03 |
| Notes | H-index: 62 |