KAGEYAMA Yoshiyuki
Assistant Professor
Chemistry becomes increasingly fun!
Department of Chemistry, Physical Chemistry
| Theme | We investigate dynamics in molecular systems, where many molecules play cooperatively. This insight is very far-from the classical chemistry. The self-organized behaviors like as the biological phenomena will be established from our research field. |
| Field | Systems Chemistry, Supramolecular Chemistry, Biomimetic Chemistry, Organic Chemistry, Chemical Physics |
| Keyword | Self-Organization (Dissipative Structure), Nonlinear Dynamics, Energy Conversion, Catalysis, Collective Dynamics of Molecules, Autonomous Functions, Soft Matter, Active Matter |
Introduction of Research
In our body, a large number of molecules work cooperatively. As the result of the cooperation, molecules show special functions that the molecule cannot realize by alone. The aim of our research is fully-artificial creation of such a “fascinating function” due to nonlinear networks in multi-molecular systems, in vitro. This novel research field is known as Systems Chemistry.
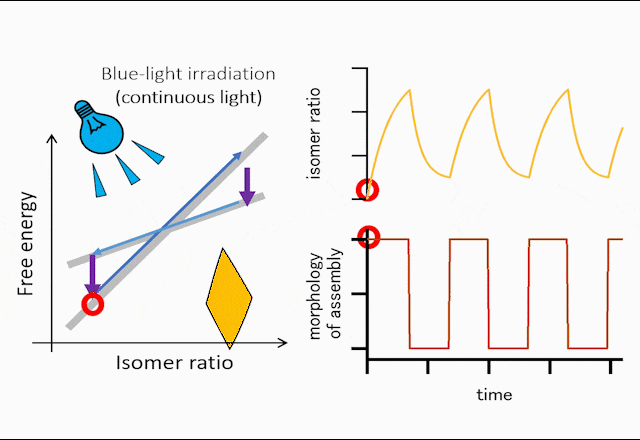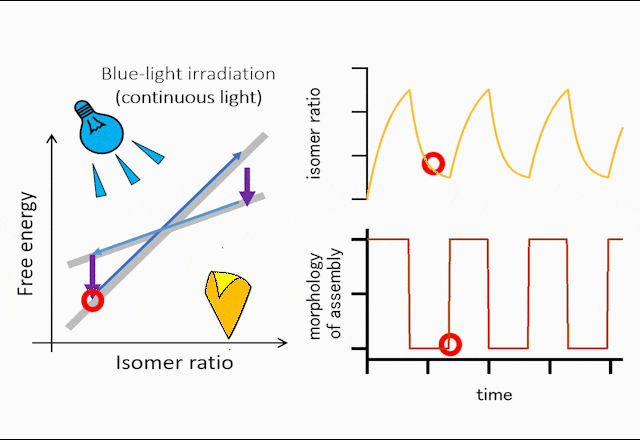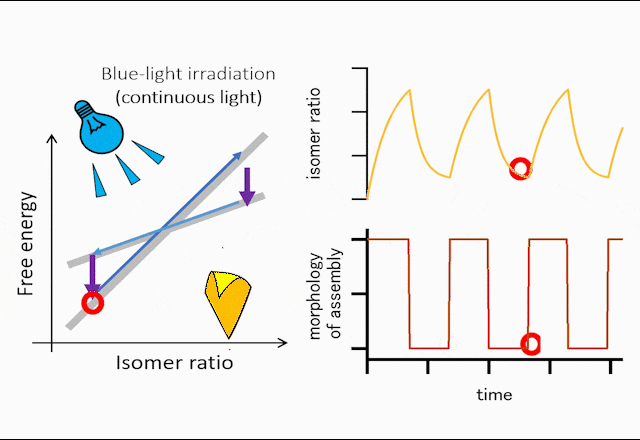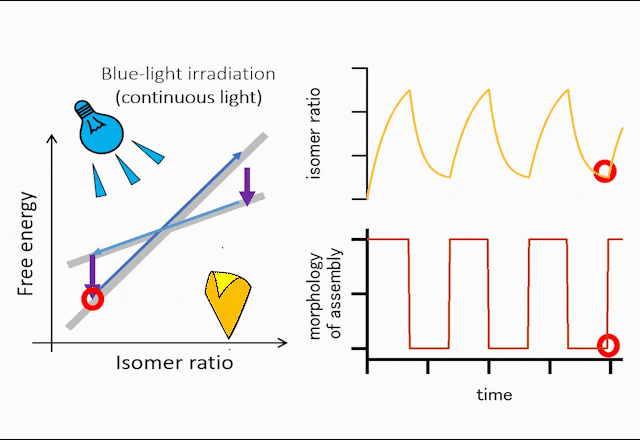
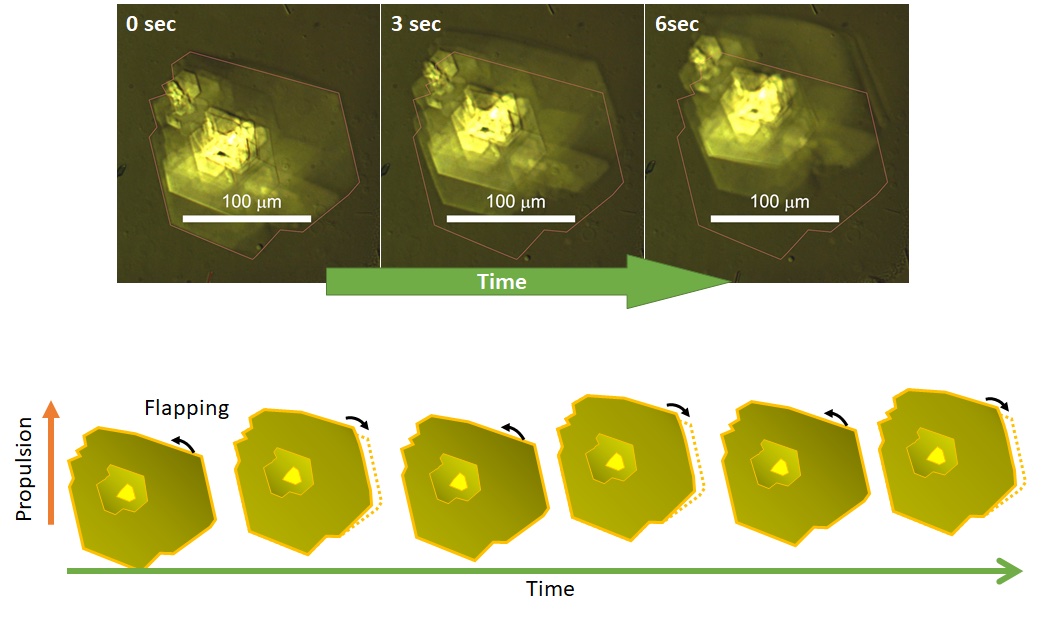
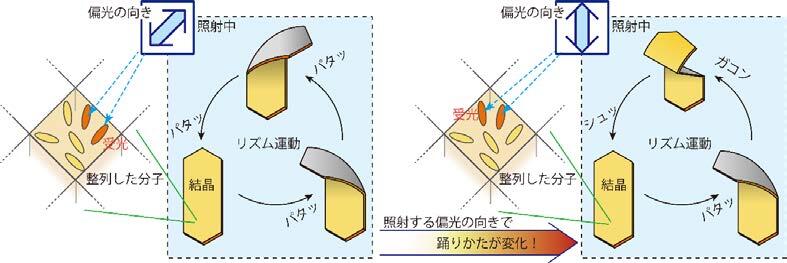
The world leading achievement is an autonomous flapper shown in Figure 1. Generally, to make the repetitive motion of light-responsive materials, switching of light is required. However, the azobenzene crystals in the figure self-continue the periodical flipping under steady light irradiation. The mechanism for the self-sustainable motion is shown in Figure 2. In the original phase of the crystal, the forward reaction (trans-to-cis photoisomerization) occurs, and the increasing in the cis-isomer ratio induces a subcritical phase transition to a metastable crystal phase. In the newly formed crystal, cis-to-trans photoisomerization occurs, and the decrease in the cis-isomer ratio induces the reverse phase transition to the original crystal phase. Repeating the dynamics, the crystal shows mechanical flips repetitively to propel in water. Hokkaido University press-released the result with the title of “On the path toward molecular robots”. The news was translated into more than ten languages by publishers. You may watch the movie of clione-like motion of crystal from Hokudai’s YouTube (Figure 3).
The field of the research group is in multidiscipline area. All who interested in the research is welcomed to join.
Representative Achievements
Y. Kageyama, et al., Cryst. Growth Des., 2025, 25, 7543-7556.
Y. Kageyama, Chem, 2025, 11, 102455
K. Obara, Y. Kageyama, S. Takeda, Small, 2022, 18, 2105302. (Press-Released Article)
T. Ikegami, Y. Kageyama, K. Obara, S. Takeda, Angew. Chem. Int. Ed., 2016, 55, 8239–8243. (Hot Article, Press-Released Article)
H. Takahashi, Y. Kageyama, K. Kurihara, K. Takakura, S. Murata, T. Sugawara, Chem. Commun., 2010, 46, 8791–8793.

Related industries
| Academic degree | Ph. D. (Multidisciplinary Science) |
| Academic background | 2001. Graduated from the University of Tokyo 2003. Obtained Master-degree from the University of Tokyo 2006. Obtained Ph.D. from the University of Tokyo 2006-2007. Postdoctral Researcher in the University of Tokyo 2007-2009. Postdoctral Researcher in Tokyo University of Science 2009- current position 2013-2017. JST PRESTO researcher |
| Affiliated academic society | Chemical Society of Japan |
| Project | "Molecular Engine", Scientific Research on Innovative Areas, KAKENHI, MEXT "Molecular Technology and Creation of New Functions", PRESTO, Japan Science and Technology Agency |
| Room address | Building 7 7-410 |