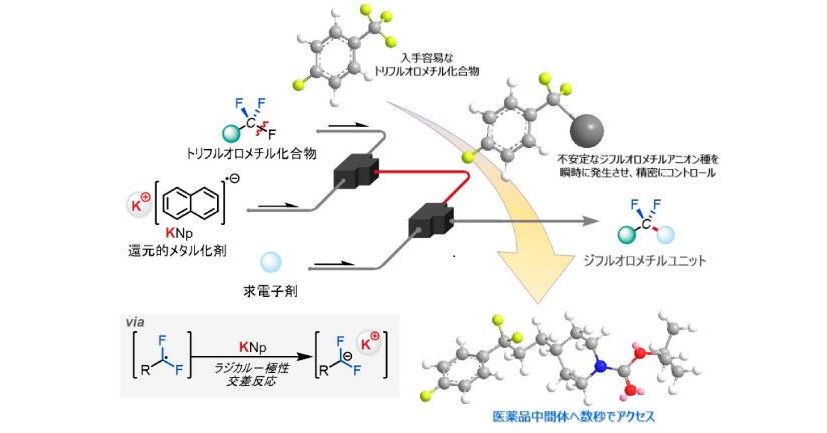
Defluorinative Functionalization Approach led by Difluoromethyl Anion Chemistry
Joint press release (in Japanese)
Abstract
Organofluorine compounds have greatly benefited the pharmaceutical, agrochemical, and materials sectors. However, they are plagued by concerns associated with Per- and Polyfluoroalkyl Substances. Additionally, the widespread use of the trifluoromethyl group is facing imminent regulatory scrutiny. Defluorinative functionalization, which converts the trifluoromethyl to the difluoromethyl motifs, represents the most efficient synthetic strategy.However, general methods for robust C(sp3)–F bond transformations remain elusive due to challenges in selectivity and functional group tolerance. Here, we present….
Read the original article on Nature Communications
Article inforamation
Kensuke Muta, Kazuhiro Okamoto, Hiroki Nakayama, Shuto Wada & Aiichiro Nagaki
Defluorinative functionalization approach led by difluoromethyl anion chemistry. Nat Commun 16, 416 (2025).
DOI:10.1038/s41467-024-52842-0