Introduction of Research
Theoretical chemistry of photo-chemical reaction
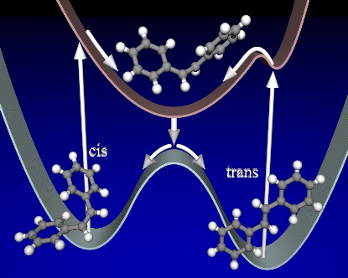
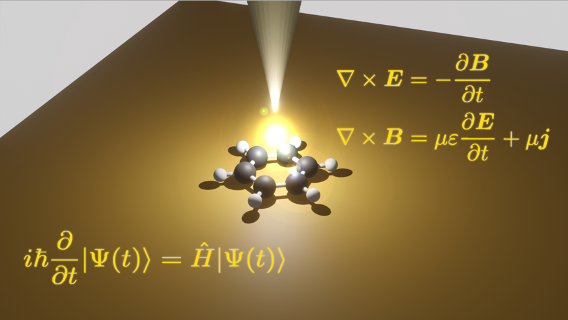
When a molecule is irradiated with UV light, the electronic state of the molecule changes drastically, causing a change in molecular structure. The chemical process in which the chemical bonding and geometrical structure change to different one is called a photochemical reaction. In the case of a fast reaction, the reaction can be completed in one trillionth of a second. The potential energy surfaces, which are determined by the electronic states, govern photochemical reactions and determine the presence or absence of light emission, the excitation lifetime, and the quantum yield. Modern theoretical chemistry uses computer calculations to evaluate these quantities to elucidate and predict mechanisms of photochemical reactions that cannot be observed in experiments.
Predicting structures, properties, and reactions of molecules with computational chemistry and data science
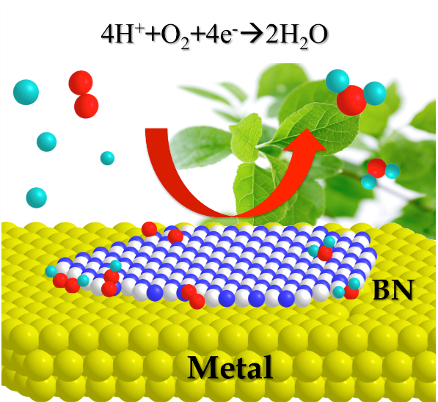
Every material consists of electrons and nuclei. Since the motions of these very small particles are governed by the Schrodinger equation, any properties of molecules can be predicted by solving this equation. But the original equation can be solved only for systems with a couple of atoms. By developing advanced computational methods that enable to treat huge molecule as well as adopting the data scientific methods such as the artificial intelligence, we are trying to elucidate and predict, for example, the catalytic reaction mechanisms and efficiency.
Theoretical and Computational Study for Nano Materials Systems
Materials at the nanoscale show novel properties that are not found in their bulk counterparts. For instance, gold is chemically very stable and therefore inert, but nano-gold shows catalytic activity to CO oxidation. Furthermore, when nano-gold is illuminated by light, a collective oscillation of electrons at the surface is excited, called localized surface plasmon, confining the light in the nanoscale. The confined, localized nano-light can also show novel properties. To explore, clarify, and understand fascinating physics and chemistry originating from these nanomaterials and nano-light, as well as the interaction between them, we are developing and applying a new theoretical framework and computational tools based on the quantum mechanics and electrodynamics.
Introduction of laboratory
In the laboratory, we don’t do experiments, but work on the computer every day. Even though students are not very good at theoretical chemistry and programming when they first join our lab, they can acquire computer skills naturally after a year without feeling any discomfort with quantum chemistry. Students with a background in quantum chemical calculations and data science are highly sought after by companies in the chemical and materials industries. Each student’s research theme is different: you can think of new theories for the first time in the world, be involved in program development, and show off the excellence of computational chemistry to your friends in experimental laboratories, all of which will make your laboratory life more meaningful.